pH Acid and Alkaline
The pH Scale
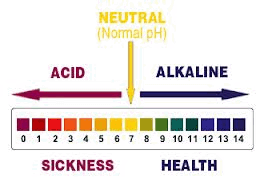
Maintaining the pH of your bodily fluids is key to health and in some cases, life itself. Digestive fluids may be as low as pH 1.0. This strong acid is needed to help break down the foods you eat. Your blood must be a slightly alkaline pH 7.35 to pH 7.45 all the time. That’s a “must,” not an “it-would-be-nice.” If the pH of your blood falls much below 7.35 or raises much above 7.45 for more than a few hours, you can’t survive.
For this test, we’re concerned with the pH of your internal environment – the potential for health of the fluids in and around your cells. When we talk about the pH of your body, we mean the pH of the fluids inside and outside your cells. The body’s ideal pH is just above pH 7.0. Similar to blood pH levels, but not quite as unforgiving, when the pH of your internal environment is too far below or too high above 7.35 to 7.45, your potential for health is compromised.
Monitoring your pH gives you an indication of how well or how hard your body is working to survive your lifestyle, the foods you eat and other stresses. The acid or alkaline level of your internal environment affects how your body functions. When your body is at its pH best, it hums along smoothly and easily. And when your body hums along smoothly and easily, your life has a good chance of doing the same. When your body is at less than its pH best, its hum may turn into an exhausted moan as it works overtime to survive. And when your body is exhausted, you are exhausted.
Neutralizing Acid Ash from Foods
Urine pH testing gives evidence of the effect your diet is having on your body. Most fruits, vegetables, and legumes are alkaline ash foods and pose no threat to the body. However, during digestion of high-protein foods such as meat, poultry, fish, grains, and nuts, the usable parts are absorbed to help nourish the body, but a residue that can’t be used is left behind. This residue is acid, and appropriately termed acid ash. We might say it’s the physiological equivalent of toxic waste. The acid of this residue can be quite strong. The residue itself will eventually make its way through the kidneys or bowel and out of the body; but before it is eliminated, it must be neutralized – weakened, buffered. If it isn’t neutralized, it can fry delicate kidney tissue.
Our bodies are smarter than we will ever be. Your own smart body has numerous ways to protect itself from an acid attack. The primary protection against strong acid is alkalizing minerals. These vital minerals can neutralize, or tone down, the acid from “quite strong” to “slightly strong.” Pretty clever. Unfortunately, in the process of neutralizing the acid, the minerals are eliminated right along with the residue. The vital neutralizing minerals tag along with the acid all the way out of your body. Gone forever. That’s the bad news.
The good news is that these lost minerals are easily replaced. Replacements come from the fruits and vegetables you eat. No problem – acid in the body is neutralized by minerals, replacement minerals come along in fruits and vegetables to take their place.
But suppose you don’t eat fruits and vegetables – well, not much, anyway.
Your intelligent body isn’t going to let a little thing like negligence keep it from doing what needs to be done. Your body is a survivor. If minerals that were lost aren’t replaced, other minerals jump in to do the job of neutralizing the acid threatening your system. But these substitute minerals weren’t just sitting on the bench waiting to be called into the game. They have important fulltime jobs, too. When they are called on to handle the emergency, they are taken from their primary jobs. For example, calcium is a “substitute” neutralizing mineral. Where do we keep our biggest calcium supply? Our bones. If you don’t replace neutralizing minerals by eating fruits and vegetables, calcium is taken from the bones. When you lose a lot of calcium from your bones, the disease label is osteoporosis, weak bones. It’s hard to live with your head held high, taking on the world, when your spine is gradually collapsing. Other minerals that make up your alkaline reserve are sodium, potassium, magnesium, and iron.
Your diet can be so top-heavy with acid ash foods that your neutralizing, or buffering systems are overwhelmed. There is just too much acid for them to handle – acid saturation. When acid-laden materials arrive at the kidneys, the kidneys must act to neutralize the acid fast, introducing another backup system: ammonia. The kidneys generate ammonia which has a pH of about 9.25 and raises the pH value considerably because it is so alkaline. Urine made alkaline by ammonia usually burns upon exit and/or has the same strong sharp smell of household ammonia.
So when your body is too acid for too long, it plays the game of life with a line of backup systems. These backups are either substitute minerals, or ammonia. When your body is too acid – when your internal pH is too low – the systems and organs of your body must work overtime just to stay even. But systems and organs aren’t designed to function in red-alert mode all the time. They need rest just as you do. If the red-alert goes on for months or years, systems and organs become exhausted. An exhausted body is no match for disease; eventually, disease wins.
What follows is a step-by-step overview of how the body neutralizes acids.
- The acid ash from many foods you regularly eat must be neutralized (buffered) before the acid is eliminated through the kidneys.
- Your vital mineral supply is used to neutralize the acid, and in the process these minerals are lost through the kidneys and bowel.
- If the neutralizing minerals aren’t replaced by eating a varied selection of quality foods, they will be taken from the body’s reserves to neutralize the acid.
- If the back-up neutralizing (buffer) systems aren’t up to the task, or if the body is saturated with acid from too much protein, the kidneys generate ammonia as a last-ditch effort.
- When the body is over-acid, buffer systems are overwhelmed, and all systems, organs, and processes are overstressed.
- The body’s systems and organs aren’t able to perform at their best because they have become exhausted.
- This exhaustion opens the door to disease.
What does all of this have to do with checking pH?
Urine pH values are your clue to whether or not your alkaline arsenal has been, or is being, used up or overwhelmed and if your ammonia backup system is taking the role of key acid neutralizer. Urine pH values indicate whether or not your body is overburdened with too much acid from too much high protein food, which creates toxicity.
Ultimately what you learn from checking your urine pH is whether or not the foods you regularly eat leave the door open to disease.
Other Sources of Acid
Your body works constantly to get rid of acid, no matter where it comes from. Acid ash-producing food isn’t the only source of acid in your body. Two other prominent sources contribute to your internal acid level: (1) cellular activity, and (2) naturally acid foods.
(1) Your cells produce acid as they function. As long as cells are alive, they work and produce acid. In addition, when you exercise, cells produce even more acid than when you are resting.
However, there’s a big difference between the acid your cells produce and the acid that you get from high-protein acid ash producing foods. In the first place, the acid from cells – physiological acid, it’s called – is a lot weaker than acid from high-protein acid ash foods. And in the second place, self-produced cellular acid doesn’t need to be neutralized by vital minerals before it is sent out of the body. Self-produced cellular acid is easily eliminated through your lungs when you breathe and when you talk.
(2) Besides cellular activity, acid is also a factor when you eat acidic fruits such as oranges and lemons. However, instead of acid fruits being a problem for your body, they contribute much needed alkalizing minerals that help to keep your internal pH under control. Acid by nature; alkaline by function.
Food Overview – NO GMOs
Some Common Acid Ash Foods
Leave strong acid in your internal environment
Red meat, pork, lamb, poultry, fish, eggs, grains (see exceptions below), lentils, dried peas, beans, dairy products including cheese and yogurt, processed, packaged and canned foods, breakfast cereal, and everything made out of flour including bread, crackers, bakery goods, and pasta. Fried food, refined salt, artificial colors and flavors, tobacco, white vinegar, coffee, soda, hard alcohol, beer, and most nuts. Chocolate, heated honey, blueberries, bottled fruit juices, cooked spinach, peeled white potatoes. Additionally, non-prescription and prescription drugs, chemicals and pesticides, stress, anger, worry, hatred, anxiety, and exhaustion are all acid-forming.
Some Common Alkaline Ash Foods
Help to control acid in your internal environment
Most fruits including avocados, tomatoes and citrus, most vegetables, goat milk products, fermented foods, almonds, brazil nuts, amaranth, millet, quinoa, buckwheat, lima beans, soy products, raw honey, maple syrup, stevia, molasses, mushrooms, and sweet potatoes. Apple cider vinegar, organic wine, sea vegetables, natural herbs and spices. Additionally, happiness, pleasure, fun, laughter, joy, peace, relaxation, and deep rest are all alkaline-forming.
Some Common Neutral Ash Foods
Leave an alkaline ash but have an acidifying effect on the body
Corn oil, corn syrup, olive oil, refined sugar, plums, prunes and cranberries.